Get exam-ready with the Class 10 Science 086 Previous Year Question Paper 2025 designed as per the latest CBSE exam pattern. Practicing previous year papers is one of the most effective ways to assess your preparation level, understand the types of questions asked, and strengthen time management skills. This resource includes the complete 2025 Science paper (Code 086) along with detailed solutions and a marking scheme to help you analyze and improve your answers. Whether you’re aiming to revise efficiently or score high in your board exams, this question paper will be a valuable tool in your study plan. Download the PDF now and take a confident step toward your success in CBSE Class 10 Science.
Series GFHE5
Question Paper Code 31/5/1 Set 1
SCIENCE
(Session 2024-25)
Time allowed : 3 hours
Maximum Marks : 80
General Instructions :Read the instructions very carefully and strictly follow them:
1. This question paper comprises 39 questions. All questions are compulsory.
2. The question paper is divided into five sections – A, B, C, D and E
3. Section A – Question Nos. 1 to 20 are multiple choice questions. Each question carries 1 mark.
4. Section B – Question Nos. 21 to 26 are very short answer type questions. Each question carries 2 marks. Answer these questions should be in the range of 30 to 50 words.
5. Section C – Question Nos. 27 to 33 are short answer type questions. Each question carries 2 marks. Answer these questions should be in the range of 50 to 80 words.
6. Section D – Question Nos. 34 to 36 are long answer type questions. Each question carries 2 marks. Answer these questions should be in the range of 80 to 120 words.
7. Section E – Question Nos. 37 to 39 are of 3 source-based/case-based units of assessment carrying 4 marks each with sub-parts.
8. There is no overall choice. However, an internal choice has been provided in some sections. Only one of the alternatives has to be attempted in such questions.
SECTION – A
Select and write the most appropriate option out of the four options given for each of the questions 1-20. There is no negative mark for the incorrect response.
1. The main observations while performing the experiment of burning magnesium ribbon in air are:
(i) Magnesium ribbon burns with a dazzling white flame
(ii) A white powder is formed
(iii) Magnesium ribbon vapourises
(iv) Aqueous solution of the white powder turns blue litmus to red
(A) (i) and (iv)
(B) (ii) and (iii)
(C) (i) and (ii)
(D) (iii) and (iv)
2. A metal, M, displaces iron from aqueous solution of ferrous sulphate but fails to do so in case of aqueous solution of aluminium sulphate. The metal M is
(A) Magnesium
(B) Copper
(C) Lead
(D) Zinc
3. A common feature observed in the crystals of washing soda, copper sulphate, gypsum and ferrous sulphate is that all
(A) exhibit basic nature
(B) exhibit acidic nature
(C) have fixed number of molecules of water of crystallisation in one formula unit of these salts.
(D) are coloured
4. A metal ‘X’ on treatment with sodium hydroxide liberates a gas ‘G’. It also liberates the same gas, ‘G’ on treatment with dilute sulphuric acid.
Based on above information, ‘X’ and ‘G’ respectively are
(A) Copper and Sulphur dioxide
(B) Zinc and Sulphur dioxide
(C) Zinc and Hydrogen
(D) Copper and Hydrogen
5. The values of a, b, c and d in the following balanced chemical equation are respectively:
a Pb(NO3)2 heat→ b PbO + c NO2 + d O2
(A) 1,1,2,1
(B) 1,1,1,2
(C) 2,2,1,4
(D) 2,2,4,1
6. During electrolytic refining of copper, the anode, the cathode and the electrolyte used respectively are
(A) Impure copper, pure copper, acidified copper sulphate solution
(B) Pure copper, impure copper, sulphuric acid
(C) Pure copper, impure copper, acidified copper sulphate solution
(D) Impure copper, pure copper, distilled water
7. If we make carbon skeleton with four carbon atoms, the two different possible skeletons will be
Ans: (B)
8. Listed below are the steps of nutrition in Amoeba. Select the correct sequence of these steps :
(i) Diffusion of simple nutrients into cytoplasm
(ii) Food vacuole formation
(iii) Formation of finger like temporary extensions of cell surface
(iv) Complex substances broken to simpler ones
(v) Undigested material thrown out of the cell surface
(A) (iv), (i), (iii), (v)
(B) (iii), (ii), (iv), (i), (v)
(C) (ii), (i), (iv), (v), (iii)
(D) (iii), (iv), (i), (ii), (v)
9. Which among the following is not a neural action controlled by the part of human brain labelled ‘X’ in the figure below?
(A) Salivation
(B) Hunger
(C) Vomiting
(D) Blood Pressure
10. The modes of reproduction in Spirogyra and Planaria respectively are
(A) Regeneration and budding
(B) Regeneration and fragmentation
(C) Fragmentation and regeneration
(D) Budding and regeneration
11. The plant hormones promoting rapid cell division in seeds and wilting of leaves respectively are
(A) Auxins and Abscisic acid
(B) Cytokinins and Abscisic acid
(C) Gibberellins and Auxins
(D) Abscisic acid and Gibberellins
12. In aerobic respiration, the steps are : breakdown of glucose to pyruvate and its further conversion to carbon dioxide. Both processes respectively occur in –
(A) Vacuole and Cytoplasm
(B) Chloroplast and Mitochondria
(C) Mitochondria and Cytoplasm
(D) Cytoplasm and Mitochondria
13. In order to obtain large images of the teeth of patients, the dentist holds the concave mirror in such a manner that the teeth are positioned
(A) at the focus of mirror
(B) between pole and focus of the mirror
(C) between focus and centre of curvature of the mirror
(D) at the centre of curvature of the mirror
14.The possible way to restore clear vision of those people whose eyeball has elongated is the use of suitable
(A) bifocal lens
(B) concave lens
(C) converging lens
(D) convex lens
15. The examples of natural and manmade (artificial) ecosystems are respectively
(A) Forests and ponds
(B) Crop fields and lake
(C) Lakes and gardens
(D) Crop fields and forests
16. Human activities that are affecting the environment are:
(A) minimising the use of chloroflurocarbons
(B) excessive use of disposable cups and plates
(C) maximising the use of reusable utensils for eating food and drinking fluids
(D) segregating the wastes into biodegradable and non-biodegradable before
disposal
For Q. Nos. 17 to 20, two statements are given — One labelled as Assertion (A) and the other labelled as Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below:
17. Assertion (A) : Silver chloride turns grey in sunlight.
Reason (R) : Decomposition of silver chloride into silver and chlorine takes place by sunlight.
(a) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A)
(b) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A)
(c) Assertion (A) is true, but Reason (R) is false
(d) Assertion (A) is false, but Reason (R) is true
18. Assertion (A) : The embryo gets nutrition from the mother’s blood with the help of a special tissue called placenta.
Reason (R) : Placenta is a disc which is embedded in the uterine wall
(a) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A).
(b) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A)
(c) Assertion (A) is true, but Reason (R) is false
(d) Assertion (A) is false, but Reason (R) is true
19. Assertion (A) : The pattern of the magnetic field of a solenoid carrying a current is similar to that of a bar magnet.
Reason (R) : The pattern of the magnetic field around a current carrying conductor is independent of the shape of the conductor.
(a) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A)
(b) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A)
(c) Assertion (A) is true, but Reason (R) is false
(d) Assertion (A) is false, but Reason (R) is true
20. Assertion (A) : All organisms can make organic compounds like sugar and starch from inorganic substances using radiant energy of the sun.
Reason (R) : The organisms which can produce food by photosynthesis are called producers.
(a) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A)
(b) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A)
(c) Assertion (A) is true, but Reason (R) is false
(d) Assertion (A) is false, but Reason (R) is true
SECTION – B
Question Nos. 21 to 26 are Very Short Answer type questions. Each question carries 2 marks.
21. A crystalline substance of green colour ‘X’ emits gases of characteristic odour when heated over a flame. It first loses water and changes colour. On further heating, it decomposes and produces a solid compound Y.
(a) Identify ‘X’ and ‘Y’
Ans: X = FeSO4 . 7H2O / Ferrous Sulphate Crystals
Y = Fe2O3/ Ferric Oxide
(b) State the change in colour observed when ‘X’ is heated
Ans: Green to white /brown
22. Give reasons :
(a) The male reproductive organ responsible for formation of germ cells is located outside the abdominal cavity.
Ans: Provides a lower temperature than the normal body temperature for sperm formation.
(b) The roles of the glands, present along the path of the vas-deferens, are very significant.
Ans: The secretion of the glands helps in the transport of sperms and provides nutrition.
23. (A) How is lymph formed? State its important function.
Ans: Through the pores present in the walls of capillaries some amount of plasma, proteins and blood cells escape into intercellular spaces in the tissue to form the tissue fluid called lymph.
Lymph carries digested and absorbed fat from intestine/ drains excess fluid from extracellular space back into the blood.
OR
(B) (a) Identify ‘X’ in the figure of human nephron shown below. What role does it play in the process of urine formation ?
Ans: X- Bowman’s capsule;
Function: collects the filtrate
(b) Why some substances are selectively reabsorbed from the initial filtrate of urine, as it flows along the tabular part of nephron?
Ans: It is because the nephron monitors how much excess water is there in the body and how much dissolved waste is to be removed or how much useful substances are retained by the body.
24. The value of absolute refractive indices of kerosene and water are 1.44 and 1.33 respectively. Compare the two media on the basis of their
(a) optical density
(b) mass density
(c) relative speed of propagation of light
What do you infer on the basis of above comparisons?
| Kerosene (1·44) | Water (1·33) |
| (a) Higher optical density | Lower optical density |
| (b) Lower mass density | Higher mass density |
| (c) Lower speed of light | Higher speed of light |
Inference: Although, kerosene is optically denser than water but its mass density is less than water/ An optically denser medium may not possess greater mass density.
25. (A) State two applications of Joule’s heating in domestic electric circuit.
Ans: Electric bulb / electric iron / Electric fuse / Electric heater / electric Oven
(B) (a) Establish the relationship between the commercial unit of electric energy and the SI unit of electric energy.
Ans: 1 kWh = 1000 watt x 3600 second
= 3·6 x 106 watt second
= 3·6 x 106 Joule (J)
OR
(b) Determine the total resistance of the parallel combination of three resistances of 2 ohm, 4 ohm and 6 ohm.
Ans:
26. (a) Why are the organisms of first trophic level important in any food chain?
Ans: The organisms of the first trophic level fix up the solar energy and makes it available for heterotrophs or the consumers.
(b) Justify the given statement: “The flow of energy in an ecosystem is unidirectional.”
Ans: As energy moves progressively through various trophic levels it is no longer available to previous level / The energy that is captured by autotrophs does not revert back to solar input. /The energy passed to herbivores does not come back to autotrophs(producers).
SECTION – C
Question Nos. 27 to 33 are Short Answer type questions. Each question carries 3 marks.
27. Write chemical formula of washing soda. How is it obtained from baking soda? List two uses of washing soda.
Ans: Na2CO3.10H2O
When baking soda is heated sodium carbonate is obtained and recrystallisation of sodium carbonate gives washing soda.
2NaHCO3 (Heat)→ Na2CO3 + H2O + CO2
Na2CO3 +10 H2O → Na2CO3.10 H2O
Uses: (i) In glass / soap / paper industry (ii) In manufacture of borax
28. (A) Observe the following diagram showing an experiment to determine the conditions under which a metal ‘M’ corrodes.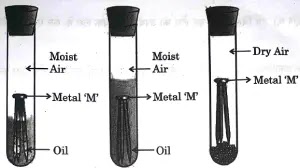
List your observations in each of the three cases A, B and C with reason, if the metal ‘M’ is generally protected against corrosion by the method of galvanisation.
Ans: A: Metal M will get corroded partly
The part of metal M outside oil will get corroded whereas the part of the metal M inside the oil will not corrode as it cannot react with moist air.
B: Metal M will not undergo corrosion.
It is inside the oil and not exposed to moist air.
C: Metal M will not undergo corrosion as moisture is absent in test tube C.
OR
(B) (a) Show the formation of Aluminium Nitride (AlN) by the transfer of electrons. [ At. no. of Al = 13; At. no. of N = 7]
Ans: (a) Al = 2, 8, 3
N = 2, 5![]()
(b) “Ionic compounds are solids and are generally brittle and break into pieces when pressure is applied.” Give reason to justify the statement.
Ans: Ionic compounds have strong force of attraction between the positive and negative ions /Strong interionic forces of attraction/Strong electrostatic forces of attraction.
29. (a) Write the name and one function of respiratory pigment found in human beings.
Ans: Haemoglobin
•To take up oxygen from lungs and carry it to tissues which are deficient in oxygen / Transports oxygen to various body parts.
(b) Why do lungs always contain a residual volume of air?
Ans: To provide sufficient time for oxygen to be absorbed and for carbon dioxide to be released.
(c) Why is ATP known as energy currency of the living beings?
Ans: ATP provides energy for various cellular processes
30. (a) Define fertilisation.
Ans: Fertilization is the fusion of male and female gametes to form zygote.
(b) What happens to Zygote, Ovule, Ovary and Stamens after fertilisation in a flowering plant?
Ans: (i) Zygote forms embryo
(ii) Ovule forms seeds
(iii) Ovary forms fruits
(iv) Stamens withers away (shrivel and falls off)
31. The power of lens is -0.25 D. Based on this information, find out
(a) The type of lens and its focal length.
Ans: Concave lens
P = 1/f(m) ⇒ f = 1/(-0.25) ⇒ f = -4m
(b) The eye defect for which it may be used as a corrective lens.
Ans: Myopia
(c) The nature and size of the image formed by this lens when an object is placed between F and 2F from the optical centre of this lens.
Ans: Virtual , Erect
Diminished
32. The resistance of a wire of 0.01 cm radius is 7 ohms. If the resistivity of the material of the wire is 44 x 10-6 ohm meter, calculate the length of the wire.

33. (a) “The third wire of earth connection is very important in domestic electric appliances.” Justify this statement.
Ans: The third wire (earth wire) is a safety measure to ensure that in case of any leakage of current to the metallic body of the appliance, it keeps its potential to that of the Earth and the user may not get a severe shock.
(b) List two precautions to be taken to avoid the overloading of domestic electric circuits.
Ans: (i) Use of electric fuse of proper rating
(ii) Not connecting too many appliances to a single socket.
SECTION – D
Question Nos. 34 to 36 are Long Answer type questions. Each question carries 5 marks.
34. (A) (a) What is meant by the term homologous series of carbon compounds? Write molecular formula of any two consecutive members of homologous series of ketones.
Ans: A series of carbon compounds in which the same functional group or hetero-atom substitutes for hydrogen in a carbon chain. A sequence of carbon compounds with same general formula and similar chemical properties.
CH3COCH3, CH3COC2H5
(b) Write chemical equation of the reactions of ethanoic acid with
(i) Sodium hydroxide
Ans: (i) CH3COOH+NaOH → CH3COONa+H2O (Sodium ethanoate /Sodium acetate)
(ii) Ethanol (in the presence of an acid); giving the name of the products in each case.
Ans: C2H5OH+CH3COOH → CH3COOC2H5 + H2O (Ester/Ethyl Ethanoate)
(c) Draw the structure of the molecule of benzene.
Ans: 
OR
(B) (a) Write the molecular formula of ethyne an draw its electron dot structure.
Ans: C2H2
(b) Write chemical equation to a the reaction of ethanol with the name of the product formed in each case.
(i) Sodium metal
Ans: 2C2H5OH + 2Na → 2C2H5ONa + H2 Sodium ethoxide
(ii) Ethanoic acid (in the presence of an acid)
Ans: 
(iii) Acidified potassium dichromate
Ans: 
35. (A) (a) Analyse the given situations and interpret the possible reason for each:
(i) Iodine deficiency in diet increases the possibility of a disease of swollen neck in a person.
Ans: Iodine is necessary for the thyroid gland to make thyroxin hormone, its deficiency causes goitre.
(ii) Some people in population men have very short heights (dwarfs). . .
Ans: Deficiency of growth hormone in childhood causes dwarfism.
(iii) Thick facial hairs develop in boys at the age of 10-12 years.
Ans: Secretion of testosterone during puberty in males.
(b) Explain two reasons which necessitate the need of chemical communication in multicellular organisms.
Ans: (i) Hormones or chemical compounds can potentially reach all cells of body steadily and persistently.
(ii) Hormones help to coordinate growth, development and responses to environment.
OR
(B) (a) Differentiate between voluntary and involuntary action.
| VOLUNTARY ACTION | INVOLUNTARY ACTION |
| Thinking is involved | Does not involve thinking |
| Controlled by Forebrain | Controlled by Hindbrain |
| It occurs according to our will | It does not occur according to our will |
(b) Define reflex action. With the help of a flow diagram, show the correct sequence of path of Nerve impulse from place of its origin.
Ans: Reflex action: Sudden action in response to stimulus in the environment.
Stimulus → Receptors → Sensory Neurons → Spinal Cord/Brain → motor neuron → Effector muscle/Gland
36. (A) (a) Observe the following diagram and compare
(i) speed of light
Ans: Speed of light in A and B is same whereas the speed of light in C is greater than that of A and B.
(ii) optical densities of the three media A, B and C. Also give justification for your answer of any one of the two cases in terms of refractive indices of A, B and C.
Ans: Optical density of A and B is same whereas optical density of C is less than that of A and B.
Since the ray of light does not bend while passing from A to B the refractive indices of A and B are same and since it bends away from the normal while passing from B to C the refractive index of C is less than that of A and B. Refractive index of a medium is inversely proportional to the speed of light in that medium.
(b) Redraw the path of a ray of light through the three media, if the ray of light starting from medium A falls on the medium B
(i) Obliquely and the optical density of medium B is made more than that of A and C.
Ans: Oblique Incidence
(ii) The ray falls normally from medium A to medium B.
Ans: Normal Incidence
OR
(B) Analyse the following observation table showing variation of image distance (v) with object distance (u) in case of a convex lens and answer the questions that follow without doing any calculations:
(a) Determine the focal length of the lens. Give reason for your answer.
Ans: 2f = 40 cm ⇒ f = 20 cm
Reason: When an object is placed at 2f (– 40 cm) of a convex lens its real image formed at 2f (+ 40 cm) on the other side of the lens.
(b) Find magnification of the image formed in Observation No. 3.
Ans: m = v/u = +60cm/-30cm = -2
(c) The numerical value of magnifications in cases of observation 1 and 2 is same. List two differences in the images formed in these two cases.
Ans: Observation No.1- image is virtual and erect
Observation No.2 – image is real and inverted
SECTION — E
Question Nos. 37 to 39 are Case/Data based questions with 2 or 3 sub parts. Internal choice is provided in one of these sub parts. Each question carries 4 marks.
37. The combining capacity of various elements depends on the number of valence electrons. Also the reactivity of elements is explained as their tendency to attain a completely filled outer shell, that is, to attain a noble gas configuration. This may be either through gain of electrons or loss of electrons or sharing of electrons.
| Observation Number | Object distance (u) in cm | Image distance (v) in cm |
|---|---|---|
| 1 | -15 | -60 |
| 2 | -25 | +100 |
| 3 | -30 | +60 |
| 4 | -40 | +40 |
| 5 | -60 | +30 |
| 6 | -100 | +25 |
(a) An element A has atomic number 16, how will it attain its nearest noble gas configuration?
Ans: It shall gain or share 2 electrons to attain its nearest noble gas configuration.
(b) Write the number of (i) single and (ii) double covalent bonds in a molecule of butene (C4H8).
Ans: (i) The number of single covalent bonds- 10
(ii)The number of double covalent bonds-1
(c) (A) Explain the formation of a molecule of ammonia (NH8), using electron dot structure. (Atomic number of nitrogen is 7)
Ans: 
OR
(c) (B) Why does carbon share its valence electrons with other atoms of carbon or with atoms of other elements?
Ans: Carbon cannot gain or lose 4 electrons to complete its octet as a large amount of energy is involved.
(i) It could gain four electrons forming C4- anion. But it would be difficult for the nucleus with six protons to hold on to ten electrons.
(ii) It could lose four electrons forming C4+ cation. But it would require a large amount of energy to remove four electrons leaving behind a carbon cation with six protons in its nucleus holding on to just two electrons.
38. In human beings, there are 23 pairs of chromosomes. Out of these 23 pairs of chromosomes (i.e. 46 chromosomes), 22 pairs of chromosomes are called autosomes and one pair of chromosomes. i.e. two chromosomes are called sex chromosomes. The sex chromosomes are of two types – ‘X’ chromosomes and ‘Y’ chromosomes. The sex of a child (i.e. progeny), is decided at the time of fertilisation. In other words, at the time of zygote formation the sex chromosomes inherited from the parents of a child decide whether the new born will be a boy or a girl.
(a) What are chromosomes?
Ans: Chromosomes carry genes which control the traits of an organism./Chromosomes contain information for inheritance of features from parents to next generation in form of DNA (deoxyribonucleic acid) molecules
(b) Why is the pair of sex chromosomes in human males called mismatched pair?
Ans: Men have one normal sized X chromosome while Y chromosome is short.
(c) (A) Show with the help of a flow chart that the statistical probability of getting a boy or a girl is 50: 50.

OR
(c) (B) Mention two examples of animals where sex is not determined genetically like human beings. Describe in brief the method of sex determination in these animals.
Ans: (i) In a few reptiles; the temperature at which fertilized eggs are kept determine the sex of offspring.
(ii) In snails; the individual can change sex, indicating that is not genetically determined.
39. In order to obtain magnetic field lines around a bar magnet, a student performed an experiment using a magnetic compass and a bar magnet. The magnet was placed on a sheet of white paper fixed on a drawing board. Using magnetic needle he obtained on the paper a pattern of magnetic field lines (as shown below) around the bar magnet.
(a) By convention, the field lines emerge from north pole and merge at south pole. Why? Give reason.
Ans: The direction of the magnetic field is taken to be the direction in which a north pole of the compass needle moves inside it.
(b) State the relationship between strength of the magnetic field and the degree of closeness of the field lines.
Ans: Closer the field lines stronger is the magnetic field.
(c) (A) (i) No two fields lines can ever intersect each other. Give reason.
Ans: It would mean that at the point of intersection, the compass needle would point towards two directions, which is not possible.
(ii) The magnetic field in a given region is uniform. Draw a diagram to represent it.
Ans: Equidistant parallel lines
OR
(c) (B) Draw the pattern of nae magnetic field lines through and around a current carrying solenoid. What does the pattern of field lines inside the solenoid represent?
Ans: Uniform Magnetic Field
Other Related Links
- Class 10 AI 417 Previous Year Question Paper 2025 with Solution
- Class 10 Artificial Intelligence 417 Previous Year Question Paper 2022 with Solution – Term 1
- Class 10 Artificial Intelligence 417 Previous Year Question Paper 2022 with Solution – Term 2
- Class 10 Artificial Intelligence 417 Previous Year Question Paper 2023 with Solution
- Class 10 Artificial Intelligence 417 Previous Year Question Paper 2024 with Solution
- Class 10 Computer Application 165 Previous Year Question Paper 2022 with Solution – Term 2
- Class 10 Computer Application 165 Previous Year Question Paper 2023 with Solution
- Class 10 Computer Application 165 Previous Year Question Paper 2024 Solution
- Class 10 Computer Application 165 Previous Year Question Paper 2025 with Solution
- Class 10 English Comm 101 Previous Year Question Paper 2025 with Solution
- Class 10 English LL 184 Previous Year Question Paper 2024 – SET 1
- Class 10 English LL 184 Previous Year Question Paper 2024 – SET 2
- Class 10 English LL 184 Previous Year Question Paper 2024 – SET 4
- Class 10 English LL 184 Previous Year Question Paper 2024 – SET 5
- Class 10 English LL 184 Previous Year Question Paper 2025 – SET 2
- Class 10 English LL 184 Previous Year Question Paper 2025 – SET 3 with Solution
- Class 10 English LL 184 Previous Year Question Paper 2025 – SET 4 with Solution
- Class 10 English LL 184 Previous Year Question Paper 2025 – SET 5
- Class 10 English LL 184 Previous Year Question Paper 2025 – SET 6
- Class 10 English LL 184 Previous Year Question Paper 2025 with Solution – SET 1
- Class 10 Hindi A 002 Previous Year Question Paper 2024 Set 1 with Solution
- Class 10 Hindi A 002 Previous Year Question Paper 2025-1
- Class 10 Hindi A 002 Previous Year Question Paper 2025-2
- Class 10 Hindi A 002 Previous Year Question Paper 2025-3
- Class 10 IT 402 Previous Year Question Paper 2019 with Solution
- Class 10 IT 402 Previous Year Question Paper 2020 with Solution
- Class 10 IT 402 Previous Year Question Paper 2022 with Solution Term 1
- Class 10 IT 402 Previous Year Question Paper 2022 with Solution Term 2
- Class 10 IT 402 Previous Year Question Paper 2023 with Solution
- Class 10 IT 402 Previous Year Question Paper 2023 with Solution (Compartment)
- Class 10 IT 402 Previous Year Question Paper 2024 with Solution
- Class 10 IT 402 Previous Year Question Paper 2025 with Solution
- Class 10 Maths Basic 241 Previous Year Question Paper 2024 with Solution
- Class 10 Maths Basic 241 Previous Year Question Paper 2025 with Solution
- Class 10 Maths Standard 041 Previous Year Question Paper 2025 with Solution
- Class 10 Science 086 Previous Year Question Paper 2024 Set 1 with Solution
- Class 10 Science 086 Previous Year Question Paper 2025 with Solution
- Class 10 Social Science 087 Previous Year Question Paper 2024 – SET 1
- Class 10 Social Science 087 Previous Year Question Paper 2024 – SET 2
- Class 10 Social Science 087 Previous Year Question Paper 2024 – SET 3
- Class 10 Social Science 087 Previous Year Question Paper 2024 – SET 4
- Class 10 Social Science 087 Previous Year Question Paper 2024 – SET 5
- Class 10 Social Science 087 Previous Year Question Paper 2025 with Solution
